AUTOMATED HEALTHCARE PACKAGING SOLUTIONS
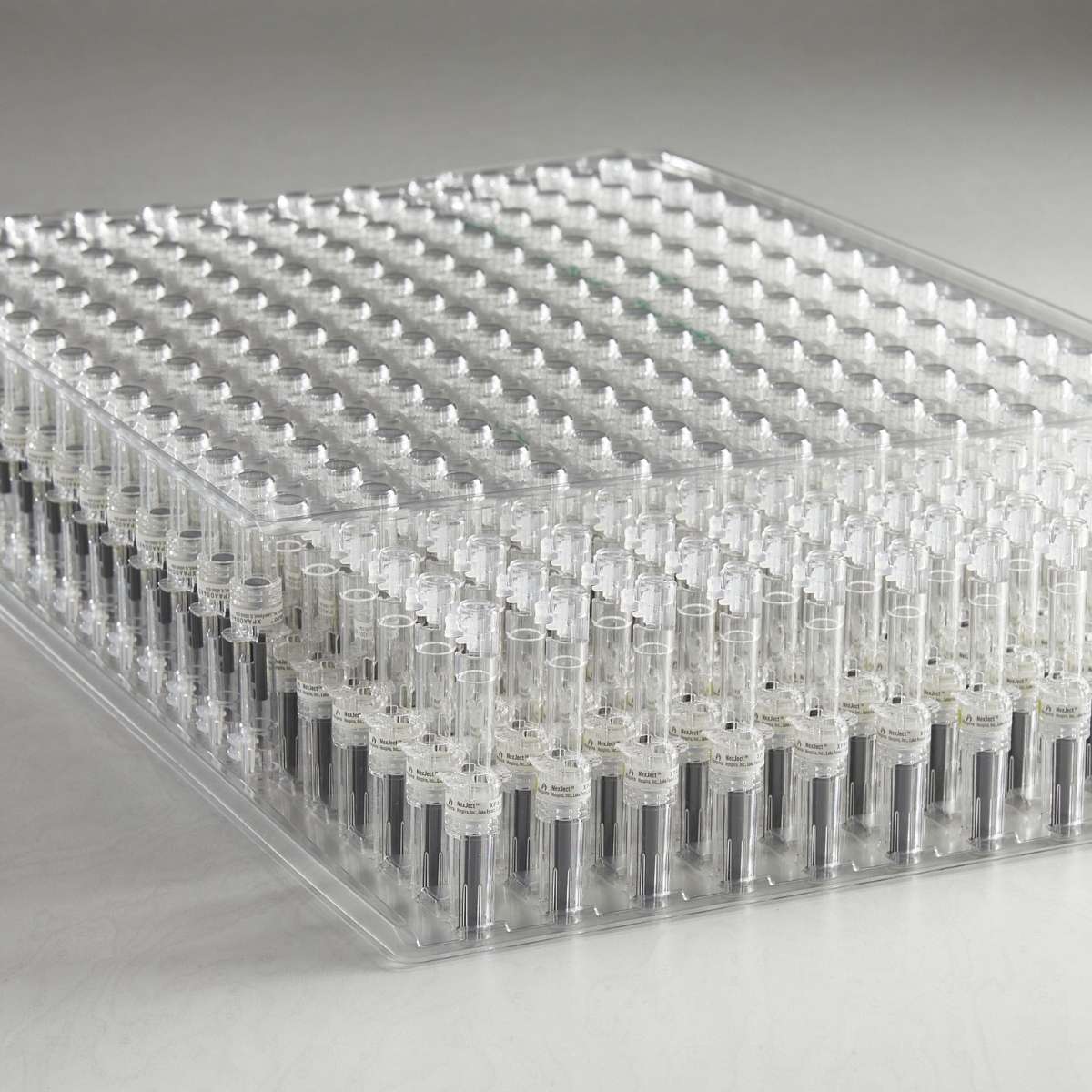
Healthcare product manufacturing, assembly, and packaging processes are becoming increasingly automated, necessitating production trays that not only protect devices during assembly and packaging but also seamlessly integrate with the automation equipment.
Plastic Ingenuity has the expertise and experience to ensure the successful design and implementation of thermoformed trays for automated packaging scenarios, no matter how complex.
When designed correctly — with collaboration from all stages of the supply chain, including the device manufacturer, automation fabricator, and packaging supplier — thermoformed trays serve as vital tools in meeting the above-mentioned goals.
INTEGRATION WITH AUTOMATION
There are several critical considerations that should be taken into account when designing thermoformed trays for integration with automation systems.
First, consider the preferred orientation and configuration of the product in the tray: Orientation is usually horizontal or vertical, while configuration can be much more complex. Making configuration and orientation decisions requires careful consultation, engineering analysis, and the weighing of a range of benefits and disadvantages. Implications for downstream processes, like stacking and cartoning, also must be considered.
It’s important to consider the different forces that a thermoformed tray will encounter during manufacturing and packaging processes. Conducting a step-by-step failure mode analysis of the automated processes allows for easier decision-making on aspects like:
▪️ Material choice, including type and gauge
▪️ Inclusion of robust design features, including ribs and chamfers
▪️ Tooling attributes to achieve ideal material distribution, including greater thicknesses in vulnerable areas
Other major considerations include matching the pitch of the end-of-arm tooling — which is critical to the efficiency of a system and requires in-depth knowledge of the automation specifications — as well as dimensional consistency, often required by automated systems.
Dimensional stability is particularly important, as the large footprint of most thermoformed trays can present unique challenges in minimizing variation. Plastic Ingenuity leverages our deep tooling design expertise, as well as our strict control measures, to achieve a high degree of dimensional accuracy in thermoformed trays for medical device automation.
DESIGN CONSIDERATION SPECIFICS
Besides the basic factors discussed above, several other more complex design considerations should also be taken into account.
Component Fit Options
▪️ The way components fit into a thermoformed tray not only impacts packing and shipping, but production as well. There are two primary types of component fits for trays — snap fit and contour fit.
▪️ Snap fit trays feature component cavities with undercuts that firmly hold the product in place. This type of fit is ideal for downstream processes and stacking and shipping, as products are very likely to be dislodged and damaged in these situations. Snap fit trays are not ideal for automated production scenarios, however, as automated picking robotics have difficulty removing components from snap fit cavities.
▪️ Like snap fit cavities, contour fit cavities are shaped like the product or components, but they lack the undercuts that secure parts in place, making these cavities ideal for automated production processes but less effective in packing or shipping operations.
Product Access
▪️ Easy product access in the form of “picks,” intentional depressions near the component cavities on thermoformed trays, is also important. When snap fit trays are used in production, picks allow access to components for robotic elements, and they do the same for end users as well. Therefore, their location and ease of use are very important.
Product Surface
▪️ Protecting the surface of products is often a high priority for medical device Protection manufacturers looking to ensure optimal branding and aesthetics, functionality, and sterility. Surface damage can occur during manufacturing, packing, and shipping and distribution, but properly designed and thermoformed trays can go a long way in providing the required protection. Specific factors to keep in mind include:
– Clearance from other components
– Clearance from other sections of the tray
– Clearance from other trays
– Size and placement of snap fit undercuts
▪️ Successful design for these and other surface protection requirements depends on clear identification of critical surfaces, at the very beginning of the thermoformed tray design process.
Stacking
▪️ Thermoformed trays are stacked and unstacked repeatedly during medical device manufacturing, throughout production and delivery stages; trays must be able to withstand the pressures of this stacking and unstacking without sacrificing component safety or stability.
▪️ Critical stacking considerations include stack height for carton fit requirements; stack- related features such as lugs, posts, ribs, and undercuts; the location of stack features for proper tray balancing; and the weight of loaded trays and how they interact with other trays.
▪️ At Plastic Ingenuity, our innovative tooling design and building technologies allow us to offer stacking features in areas that other packaging manufacturers cannot.
Material Selection
▪️ A wide variety of materials are used for thermoformed trays, the most common being polyethylene terephthalate (PET), PET glycol (PETG), and high-impact polystyrene (HIPS). The tray material best suited for your project will depend on:
– Whether the trays are meant for single- or multiple-use
– Whether they will be washed, and how
– Whether they will be disposed of or recycled (in the case of single-use trays)
– Whether they must provide electrostatic protection for the components
– Whether they are suitable for sterilization processes
Tray Consolidation
▪️ Depending on the specific process, which components are required when, and various other factors, some medical devices and medical device production methods will require several different automation trays. With a wealth of thermoformed tray design experience, Plastic Ingenuity can help you determine how many unique trays a process requires and whether they can be consolidated. Additionally, our packaging engineers can assist with tray configuration and annual tray usage estimates.
PARTNER WITH PLASTIC INGENUITY
Working with an experienced partner who can take a leadership role in your automation integration process is the key to unlocking your team’s success. Plastic Ingenuity is that partner. Working closely with medical device, combination product, and injectable delivery device manufacturers, production automation fabricators, packaging automation fabricators, and any other involved third parties, our team works hard to ensure seamless integration, every step of the way.
Working on a medical device or pharmaceutical packaging project? The team of experts at Plastic Ingenuity is here to guide you through the entire process, from design to production. Contact us today to discuss your needs with an expert.