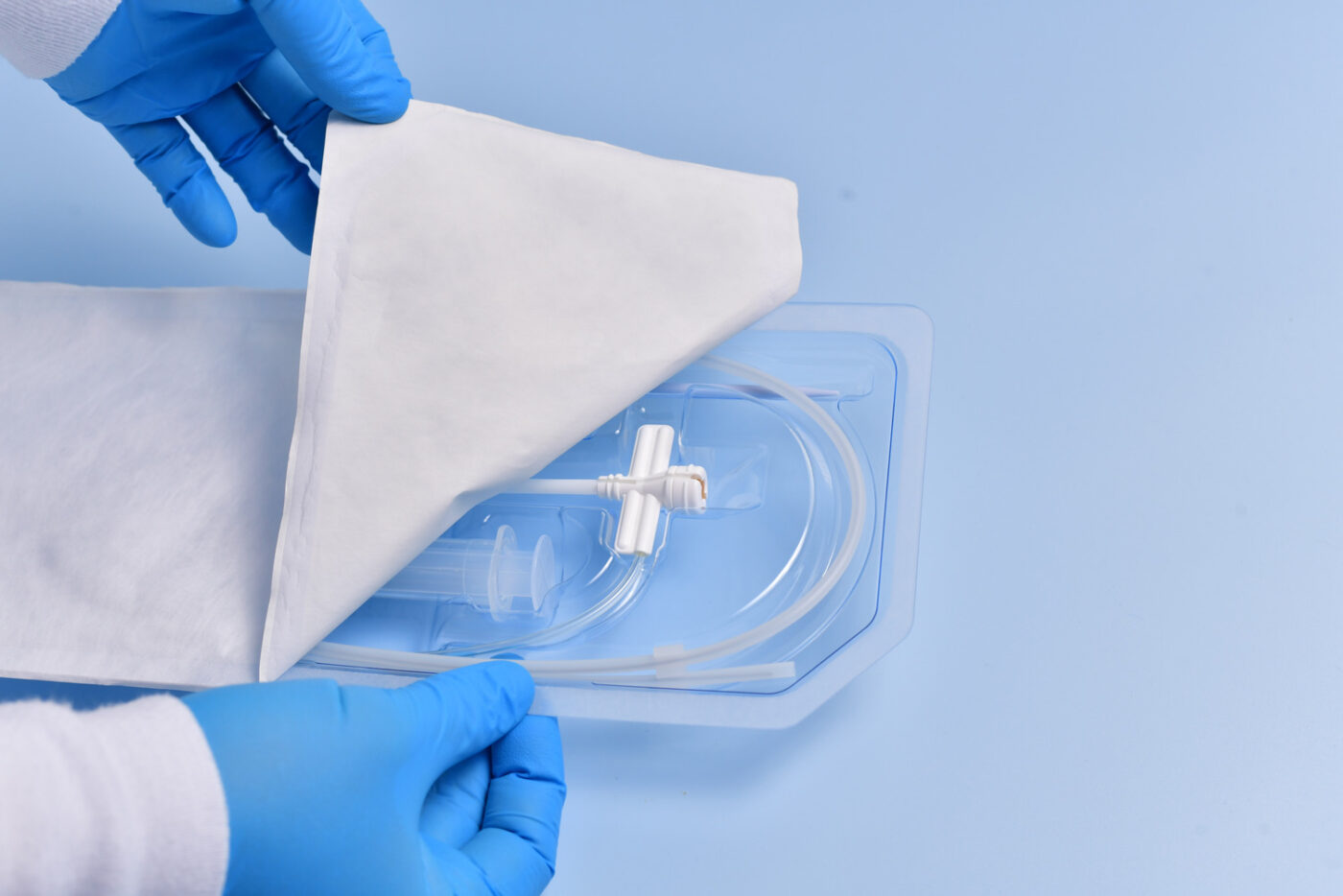
Patient safety is always the top priority at Plastic Ingenuity when working on medical device projects. From surgical instruments to implantable devices, these products must be manufactured to the highest quality standards and meet strict regulatory requirements to ensure patient safety. The design of these devices must consider a range of factors, including material selection, manufacturing processes, packaging design and validation, and quality management systems to maintain sterility.
Packaging plays a crucial role in ensuring the sterility of medical devices. Proper packaging design is essential for protecting the device from contamination during transportation, storage, and handling. Plastic Ingenuity uses various materials, including PET, PETG, HIPS, and more, to create durable, tamper-evident packaging, and can maintain sterility for extended periods.
Quality and regulatory requirements for sterile medical devices are stringent and complex. These requirements ensure that devices are safe and effective for their intended use. Manufacturers must comply with various regulations, including ISO 13485, FDA regulations, and the European Medical Device Regulation (MDR), to bring their products to market. Adherence to these standards requires meticulous attention to detail throughout the design and manufacturing process, including design control, risk management, and quality system documentation.
Let’s take a closer look at how the design, manufacturing, and quality requirements shape the outcome of a sterile barrier medical device.
MATERIAL SELECTION
Here are a few primary considerations for material selection:
- Biocompatibility: The material must be biocompatible, meaning it does not cause a toxic, allergic, or immune response in the patient.
- Sterilization compatibility: The material must withstand the sterilization process without degradation, discoloration, or other changes that could compromise its performance.
- Chemical resistance: The material should resist the chemicals used in the sterilization process and any chemicals it may encounter during use.
- Physical properties: The material must have appropriate physical properties such as strength, stiffness, and flexibility to withstand the intended use and handling.
- Manufacturing process compatibility: The material must be manufactured into the desired shape and size using appropriate manufacturing processes.
- Cost: The material selected should be cost-effective.
DESIGN
Now that material selection has been addressed; the next consideration lies within the actual design of the sterile barrier system into the parameters of the medical device itself. The sterile barrier system is designed to maintain the sterility of the device from when it is sterilized until the end user consumes it. The following are the primary considerations for designing an effective sterile barrier system:
- Packaging materials: The packaging material must withstand the sterilization process without compromising the integrity of the packaging. Common packaging materials include medical-grade roll stock, plastic films, and laminates.
- Sealing method: The packaging must be sealed to ensure an airtight and moisture-proof seal. Common sealing methods include heat sealing, adhesive sealing, and ultrasonic sealing.
- Sterilization method: The packaging must be compatible with the chosen sterilization method. Standard sterilization methods include ethylene oxide gas sterilization, steam sterilization, and gamma irradiation.
- Sterility maintenance: The packaging must be designed to maintain sterility until the device is used. This may include peelable seals, tamper-evident indicators, and sterile barriers within the packaging.
- Labeling: The packaging must be clearly labeled with information such as the device’s name, lot number, expiration date, and sterilization date. The labeling must also indicate if the packaging has been tampered with or compromised.
- Usability: The packaging must be designed for ease of use by the end user. This may include features such as tear notches, instructions for use, and compatibility with standard sterilization equipment.
- Regulatory requirements: The design of the sterile barrier system must comply with all relevant regulatory requirements for medical devices, including standards such as ISO 11607.
An effective sterile barrier system design requires careful consideration of all these factors to maintain the device’s sterility until the end user uses it. Consulting with a packaging and regulatory expert can help make informed decisions regarding sterile barrier system design.

Design for sustainability and end usability is becoming increasingly important in the design of sterile medical devices. Here are some considerations for designing sustainable and user-friendly aseptic presentations of sterile medical devices:
- Material selection: Using sustainable and biodegradable materials for packaging and product design can reduce the product’s environmental impact.
- Design for recyclability: Designing the packaging for recyclability can reduce waste and increase sustainability.
- Reduce packaging size: Minimizing the packaging size can reduce waste and transportation costs, contributing to sustainability.
- Design for ease of use: The aseptic presentation of the device should be designed with ease of use in mind. The user should be able to open and handle the packaging without compromising the device’s sterility.
- Consider usability factors: The design of the packaging and device should consider the user’s ability to manipulate the packaging, open the device, and use the device effectively.
- Clear labeling: Labeling should be clear and concise, with information on properly using and disposing of the device. This information should also include any recycling instructions.
- Design for supply chain sustainability: Consideration should be given to the supply chain’s environmental impact, including the product’s transportation and disposal.
- Consider end-of-life options: The design should consider end-of-life options for the package and device, such as recycling or proper disposal.
By considering sustainability and end usability in the design of sterile medical devices, designers can create products that meet regulatory requirements and contribute to a more sustainable and user-friendly healthcare industry.
VALIDATION
Packaging and labeling process validation is critical in manufacturing packaging for sterile medical devices. Here are three key aspects of process validation:
- Sealing equipment validation: The sealing equipment used to create the sterile barrier must be validated to produce an airtight and moisture-proof seal consistently. ASTM F2096-11 (Standard Test Method for Detecting Gross Leaks in Packaging by Internal Pressurization) and ASTM F1929-12 (Standard Test Method for Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration) are commonly used to validate sealing equipment.
- Integrity testing: The sterile barrier must be tested to ensure that it is intact and has not been compromised during manufacturing. ASTM F1929-12 (Standard Test Method for Detecting Seal Leaks in Porous Medical Packaging by Dye Penetration) and ASTM F3039-15 (Standard Test Method for Detecting Leaks in Nonporous Medical Packaging by Dye Penetration) are commonly used to validate the integrity of the sterile barrier.
- Labeling process validation: The labeling process must also be validated to ensure that the label contains all required information, is legible, and does not interfere with the integrity of the sterile barrier. ASTM F2252-03 (Standard Practice for Evaluating Ink or Coating Adhesion on Medical Device Packaging) and ASTM F2824-10 (Standard Test Method for Mechanical Seal Strength Testing for Round Cups and Bowl Containers with Flexible Peelable Seals) are commonly used to validate the labeling process.
In summary, packaging and labeling process validation involves ensuring the sealing equipment consistently produces an airtight and moisture-proof seal, testing the integrity of the sterile barrier, and validating the labeling process to ensure it meets all requirements. ASTM test methods are commonly used to validate these aspects of the manufacturing process.
QUALITY
Sterile medical device manufacturers must implement a quality management system (QMS) to consistently ensure their products meet customer and regulatory requirements. The most widely recognized QMS standard is ISO 13485:2016, which specifies requirements for the design, development, production, and distribution of medical devices.
- Design and development: Sterile medical device manufacturers must adhere to strict design and development requirements to ensure safe and effective products. The FDA’s Design Control Guidance for Medical Device Manufacturers guides the design and development process, including the design input, output, review, verification, and validation.
- Packaging and labeling: Sterile medical devices must be packaged and labeled in accordance with regulatory requirements to ensure that the device remains sterile until it is used. The FDA’s Packaging and Labeling Control Guidance for Medical Devices guides the requirements for packaging and labeling medical devices.
- Sterilization: Sterilization is a critical step in the manufacturing process for sterile medical devices. Manufacturers must ensure that their sterilization process is effective and does not compromise the safety and efficacy of the device. The most widely recognized standard for sterilization is ISO 11135:2014, which specifies requirements for the validation and routine control of ethylene oxide sterilization.
- Regulatory requirements: Sterile medical devices must comply with various regulatory requirements, including those from the FDA, the European Medicines Agency (EMA), and other regulatory agencies worldwide. In the United States, the FDA regulates medical devices under the Federal Food, Drug, and Cosmetic Act and the Medical Device Amendments of 1976. In Europe, medical devices are regulated under the Medical Devices Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR).

In summary, the manufacturing of sterile medical devices is subject to strict quality and regulatory requirements and standards to ensure that the devices are safe and effective. These requirements include implementing a quality management system, adherence to design and development requirements, compliance with packaging and labeling requirements, effective sterilization, and compliance with regulatory requirements. Plastic Ingenuity is well-versed in all facets of medical device sterility processes. For more information, please contact us, or learn more about our healthcare packaging capabilities.